(Chemistry 2023) Model past questions and answers
paragraph
I sincerely welcome you to an online platform like this. This is poscholar, and we are committed
to giving students, Jambites, undergraduate, and the likes information that will be of immense
benefit to them as there journey in thier academics.
paragraph
Recommended:
Did you choose chemistry among your subject combination for your UTME exams? if yes, then you
are in the right place because we have happily compiled UTME past question and answers for
Chemistry Jamb. We believe this will aid your learning as you get to familiarise yourself with
the patterns.
paragraph
You can study the 40 (forty) Jamb Chemistry past questions and answers for 2023 by checking
the content below or using the table of content below to navigate to the number of your choice
paragraph
paragraph
Jamb/UTME Chemistry (2023) Questions and Answers 1 - 10
1. Which of the following elements will exhibit the most electronegative character?
paragraph
(a) Al
paragraph
(b) Si
paragraph
(c) P
paragraph
(d) S
paragraph
The correct answer is: (d) S
paragraph
2. Acid hydrolysis of nitriles will yield?
paragraph
(a) Alkanes
paragraph
(b) Aldehydes
paragraph
(c) Carboxylic acids
paragraph
(d) Alcohols
paragraph
The correct answer is: (c) Carboxylic acids
paragraph
3. How many isomers of C4H9OH will be tertiary alkanol?
paragraph
(a) 1
paragraph
(b) 2
paragraph
(c) 3
paragraph
(d) 4
paragraph
The correct answer is: (b) Resonance frequency
paragraph
4. Which of the following makes oils distinguished from fats?
paragraph
(a) Oil contains a higher proportion of unsaturation
paragraph
(b) Fats contain a higher proportion of unsaturation
paragraph
(c) Oil and fats have the same proportion of unsaturation
paragraph
(d) Neither oil nor fats have unsaturation
paragraph
The correct answer is: (a) Oil contains a higher proportion of unsaturation
paragraph
5. The characteristic crystalline shape of solid water is due to _____
paragraph
(a) Covalent bonds
paragraph
(b) Hydrogen bonds
paragraph
(c) Ionic bonds
paragraph
(d) Metallic bonds
paragraph
The correct answer is: (b) Hydrogen bonds
paragraph
6. The chemical formula for laughing gas is _____
paragraph
(a) NO
paragraph
(b) NO2
paragraph
(c) N2O
paragraph
(d) N2O5
paragraph
The correct answer is: (c)
N2O
paragraph
7. The sulphide used in coating electric fluorescent tubes is _____
paragraph
(a) Iron (II) sulphide
paragraph
(b) Sphalerite
paragraph
(c) Zinc sulphide
paragraph
(d) Sulphide mineral
paragraph
The correct answer is: (c) Zinc sulphide
paragraph
8. Calculate the volume in dm3 of oxygen evolved at s.t.package. When a curent of 5A is passed throught acidified water for 772s (molar volume of gas at stp. = 22.4 dm3)
paragraph
(a) 0.056
paragraph
(b) 0.224
paragraph
(c) 224000
paragraph
(d) 56000
paragraph
The correct answer is: (b) 0.224
paragraph
9. Hydrogenation of Benzene to cyclohexane is called
paragraph
(a) Cracking
paragraph
(b) Polymerisation
paragraph
(c) Reforming
paragraph
(d) Aromatization
paragraph
The correct answer is: The examiner had aromatization in mind but it does not apply
paragraph
10. The pollutant that contributes to the depletion of the ozone layer is _____
paragraph
(a) CF
paragraph
(b) CS
paragraph
(c) CCl
paragraph
(d) CFC
paragraph
The correct answer is: (d) CFC
paragraph
Jamb/UTME Chemistry (2023) Questions and Answers 11 - 20
paragraph
11. What changes sodium hydroxide pellets to liquid?
paragraph
(a) Oxygen
paragraph
(b) Water vapour
paragraph
(c) Carbon (IV) oxide
paragraph
(d) Nitrogen
paragraph
The correct answer is: (b) Water vapour
paragraph
12. The compound used as 'antifreeze' in car radiators in cold regions of the world is _____
paragraph
(a) Ethanol
paragraph
(b) Ethylene glycol
paragraph
(c) Ethanal
paragraph
(d) Propan-1,2-diol
paragraph
The correct answer is: (b) Ethylene glycol
paragraph
13. What are the laws that form the general gas equation?
paragraph
(a) Boyle's and Charles' laws
paragraph
(b) Boyle's Charles' and Graham's laws
paragraph
(c) Gaps between the coils
paragraph
(d) The area of the coil
paragraph
The correct answer is: (c) Gaps between the coils
paragraph
14. Chlorine, bromine, and iodine resemble each other in that all
paragraph
(a) Dissolve in alkalis
paragraph
(b) Displace each other from solutions of their salts
paragraph
(c) React violently with hydrogen on heating
paragraph
(d) Are electron donors
paragraph
The correct answer is: (a) Dissolve in alkalis
paragraph
15. The method suitable for separating suspended particles in liquid is _____
paragraph
(a) Decantation
paragraph
(b) Distillation
paragraph
(c) Centrifugation
paragraph
(d) Chromatography
paragraph
The correct answer is: (c) Centrifugation
paragraph
16. A hydrocarbon X has a molar mass of 26 and a carbon atom percentage of 92.3%. What is its molecular formula?
paragraph
(a) C2H2
paragraph
(b) C2H6
paragraph
(c) CH4
paragraph
(d) C3H8
paragraph
The correct answer is: (a)
C2H2
paragraph
17. Silica gel, when exposed to air, turns liquid. What kind of substance is it?
paragraph
(a) Deliquescent
paragraph
(b) Hygroscopic
paragraph
(c) Efflorescent
paragraph
(d) Entropy
paragraph
The correct answer is: (a) Deliquescent
paragraph
18. In an equilibrium reaction, which of the following conditions indicate that the maximum yield of the product will be obtained?
paragraph
(a) Equilibrium constant is very large
paragraph
(b) △H=T△S
paragraph
(c) ΔH≥TΔS
paragraph
(d) Equilibrium constant is less than zero
paragraph
The correct answer is: (a) Equilibrium constant is very large
paragraph
19. Under high pressure, real gases do not obey laws because their molecules
paragraph
(a) Have become more energetic
paragraph
(b) Have become less energetic
paragraph
(c) Have become smaller in size
paragraph
(d) Have become larger in size
paragraph
The correct answer is: (b) Have become less energetic
paragraph
20. The salt responsible for temporary hardness is _____
paragraph
(a) Calcium sulphate
paragraph
(b) Magnesium chloride
paragraph
(c) Calcium bicarbonate
paragraph
(d) Magnesium sulphate
paragraph
The correct answer is: (c) Calcium bicarbonate
paragraph
Jamb/UTME Chemistry (2023) Questions and Answers 21 - 30
paragraph
21. Common salt (Nacl) is used to preserve foods. Which of the following properties can be used to determine its purity before use?
paragraph
(a) Solubility in water
paragraph
(b) Melting point
paragraph
(c) Relative density
paragraph
(d) Crystalline nature
paragraph
The correct answer is: (b) Melting point
paragraph
22. The rate of a chemical reaction is NOT affected by one of these factors
paragraph
(a) Color
paragraph
(b) Concentration
paragraph
(c) Presence of light
paragraph
(d) Surface area
paragraph
The correct answer is: (a) Color
paragraph
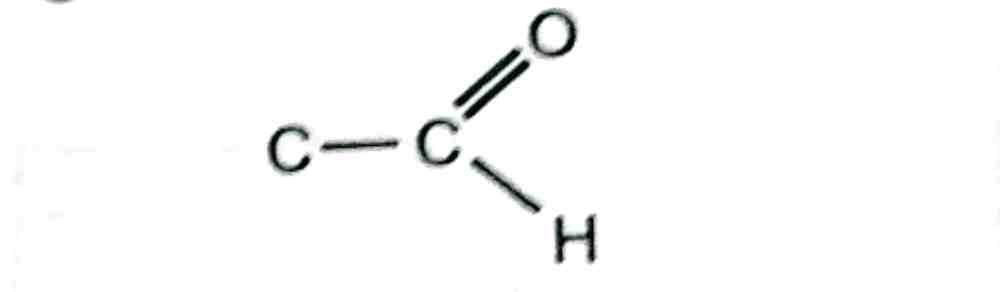
23. The functional group represented in the compound below is _____
paragraph
paragraph
(a) Alkanol
paragraph
(b) Alkanal
paragraph
(c) Alkanone
paragraph
(d) Alkanoate
paragraph
The correct answer is: (b) Alkanal
paragraph
24. On exposure to the atmosphere, a hydrated salt loses its water of crystallization to become anhydrous. This phenomenon is referred to as _____
paragraph
(a) Efflorescence
paragraph
(b) Deliquescence
paragraph
(c) Hydroscopy
paragraph
(d) Hydrolysis
paragraph
The correct answer is: (a) Efflorescence
paragraph
25. Which of the following statements is true of the electrochemical series?
paragraph
(a) Electropositivity of metals increases down the series
paragraph
(b) Electropositivity of non-metals decreases down the series
paragraph
(c) Electronegativity of non-metals decreases
paragraph
(d) Electropositivity of metals decreases down the series
paragraph
The correct answer is: (d) Electropositivity of metals decreases down the series
paragraph
26. Which of the following pairs are both substances deliquescent?
paragraph
(a) CaCl2 and H2SO4
paragraph
(b) NaOH and MgSO4.7H2O
paragraph
(c) CaCl2 and NaOH
paragraph
(d) CuO and NaCl
paragraph
The correct answer is: (c)
CaCl2 and NaOH
paragraph
27. The bond formed between H2O and H+ to form the hydroxonium H3O+ is
paragraph
(a) Dative
paragraph
(b) Covalent
paragraph
(c) Electrovalent
paragraph
(d) Ionic
paragraph
The correct answer is: (a) Dative
paragraph
28. Gas molecules are said to be perfectly elastic because
paragraph
(a) They collide without loss of energy
paragraph
(b) They move about in straight lines
paragraph
(c) The distances between them are neglible
paragraph
(d) The volume occupied by them is negligible
paragraph
The correct answer is: (a) They collide without loss of energy
paragraph
29. A solid that absorbs water from the atmosphere and forms an aqueous solution is _____
paragraph
(a) Hydrophilic
paragraph
(b) Efflorescent
paragraph
(c) Deliquescent
paragraph
(d) Hygroscopic
paragraph
The correct answer is: (c) Deliquescent
paragraph
30. Which of the following can undergo both addition reaction and substitution reaction?
paragraph
(a) Benzene
paragraph
(b) Pentane
paragraph
(c) Propane
paragraph
(d) Hexane
paragraph
The correct answer is: (a) Benzene
paragraph
Jamb/UTME Chemistry (2023) Questions and Answers 31 - 40
paragraph
31. Naming of the organic compound CH3(CH2)4CH2CH2NH2
paragraph
(a) Pentaneamine
paragraph
(b) Heptanamine
paragraph
(c) Hexanamine
paragraph
(d) Octanamine
paragraph
The correct answer is: (b) Heptanamine
paragraph
32. 2Cl(aq)− = Cl2(g) + 2e(eq)−
paragraph
The above half-cell reaction occurring at the anode during the electrolysis of dilute ZnCl2 solution is?
paragraph
(a) Ionization
paragraph
(b) Oxidation
paragraph
(c) Reduction
paragraph
(d) Recombination
paragraph
The correct answer is: (b) Oxidation
paragraph
33. CHCl3+Cl2→HCL+CCl4
paragraph
The reaction above is an example of?
paragraph
(a) An addition reaction
paragraph
(b) A decomposition reaction
paragraph
(c) A substitution reaction
paragraph
(d) A condensation reaction
paragraph
The correct answer is: (c) A substitution reaction
paragraph
34. cxHy+4O2=3Co2+2H2O. The hydrocarbon, CxHy, in the reaction above is?
paragraph
(a) Butene
paragraph
(b) Butane
paragraph
(c) Butyne
paragraph
(d) Butanone
paragraph
The correct answer is: (b) Butane
paragraph
35. One method of driving the position of equilibrium of an endohermic reaction forward is to?
paragraph
(a) Increase temperature at constant pressure
paragraph
(b) Decrease pressure at constant temperature
paragraph
(c) Cool down the apparatus with water
paragraph
(d) Decreases temperature at constant pressure
paragraph
The correct answer is: (a) Increase temperature at constant pressure
paragraph
36. 0.92 of ethanol when burned raised the temperature of 50g of water by 28.6K. Calculate the heat of combustion of ethanol. [C = 12, O = 16, H = 1, specific heat capacity of water = 4.2Jg−1K−1]
paragraph
(a) +3000KJmol−1
paragraph
(b) +300KJmol−1
paragraph
(c) −300KJmol−1
paragraph
(d) −3000KJmol−1
paragraph
The correct answer is: (c)
−300KJmol−1
paragraph
37. The repeating unit in natural rubber is _____
paragraph
(a) Alkyne
paragraph
(b) Isoprene
paragraph
(c) n-propene
paragraph
(d) neoprene
paragraph
The correct answer is: (b) Isoprene
paragraph
38. What current in amperes will deposit 2.7g of aluminium in 2 hours? [Al = 27, F = 96500Cmol−1]
paragraph
(a) 32
paragraph
(b) 8
paragraph
(c) 4
paragraph
(d) 16
paragraph
The correct answer is: (c) 4
paragraph
39. Steam changes the colour of anhydrous cobalt (II) choride from
paragraph
(a) Blue to pink
paragraph
(b) Red to white
paragraph
(c) White to green
paragraph
(d) White to blue
paragraph
The correct answer is: (a) Blue to pink
paragraph
40. Which of the following will change when a catalyst is added to a chemical reaction?
paragraph
(a) The activation energy
paragraph
(b) The potential energy of the reactants
paragraph
(c) The heat of the reaction
paragraph
(d) The potential energy of the product
paragraph
The correct answer is: (a) The activation energy
paragraph
Without doubt I believe you are happy with the questions and answers on Jamb Chemistry 2023 you
saw above. We have compiled this to ensure students have access to resources that can help them
blast their UTME exams.
If you have any comment or you observe any flaw do well to leave a
comment in the comment-box below and we will get back to you
paragraph
I recommend you check my article on the following: