WASSCE objective, essay, and practical past questions and answer (CHEMISTRY 2022)
paragraph
Great mind! welcome to poscholars website. Probably you used any of the search engine and you find us. You are welcome too.
In this post, we are going to provide you with the Wassce resource of the 2022 examination. These includes the objectives
questions and answers, the paper 2 questions and answer, and lastly the paper 3 questions and answers.
paragraph
You can study the 50 (fifty) objectives, theory and practical WASSCE physics past questions and answer for 2023 by checking
the content below or using the table of content below to navigate to the number of your choice
paragraph
Table of Contents
- WASSCE/WAEC Chemistry (2022) Questions and Answers 1 - 10
- WASSCE/WAEC Chemistry (2022) Questions and Answers 11 - 20
- WASSCE/WAEC Chemistry (2022) Questions and Answers 21 - 30
- WASSCE/WAEC Chemistry (2022) Questions and Answers 31 - 40
- WASSCE/WAEC Chemistry (2022) Questions and Answers 41 - 50
- Download WASSCE/WAEC Chemistry pdF (2023) Paper 2 (essay questions and answer 1 - 5)
- Download WASSCE/WAEC Chemistry pdf (2023) Paper 3 (Practical questions and answer 1 - 3)
paragraph
WASSCE/WAEC Chemistry (2022) Questions and Answers 1 - 10
paragraph
1. The by-product of fermentation of sugar is
paragraph
(a) Carbon (IV) oxide
paragraph
(b) Ethanoic acid
paragraph
(c) Propanol
paragraph
(d) Propan-1,2, 3-triol
paragraph
The correct answer is: (a) Carbon (IV) oxide
paragraph
2. Which of the following sugars is a product of the condensation of monosaccharides?
paragraph
(a) Galactose
paragraph
(b) Maltose
paragraph
(c) Glucose
paragraph
(d) Fructose
paragraph
The correct answer is: (b) Maltose
paragraph
3. The cleansing effect of soap is low in acidic water because of
paragraph
(a) The formation of unionized fatty acid
paragraph
(b) Increase in the PH of the soap molecules
paragraph
(c) Anti-biodegradable effect of hydrogen ions
paragraph
(d) The hardness of the acidic water
paragraph
The correct answer is: (a) The formation of unionized fatty acid
paragraph
4. The following compounds are condensation polymers except
paragraph
(a) Nylon
paragraph
(b) Protein
paragraph
(c) Starch
paragraph
(d) Polyethene
paragraph
The correct answer is: (d) Polyethene
paragraph
5. What amount of electricity is required to deposit one mole of aluminium from a solution of ?
paragraph
(a) One faraday
paragraph
(b) One ampere
paragraph
(c) Three faradays
paragraph
(d) Three amperes
paragraph
The correct answer is: (c) Three faradays
paragraph
6. Which of the following compounds would react rapidly with bromine?
paragraph
(a) Benzene
paragraph
(b) Hexane
paragraph
(c) Hexene
paragraph
(d) Hexanol
paragraph
The correct answer is: (c) Hexene
paragraph
7. Alkanols can be manufactured from alkenes by the initial reaction of alkenes with
paragraph
(a) Bromine in tetrachloromethane
paragraph
(b) Concentrated tetraoxosulphate (VI) acid
paragraph
(c) Aqueous potassium tetraoxomanganate (VII)
paragraph
(d) Sodium hydroxide solution
paragraph
The correct answer is: (b) Concentrated tetraoxosulphate (VI) acid
paragraph
8. Which of the following statements about the standard hydrogen electrode is not correct?
paragraph
(a) The hydrogen gas is at a pressure of 1 atmosphere
paragraph
(b) A solution containing 1 mol ions is used
paragraph
(c) A platinum electrode is used
paragraph
(d) The temperature is kept at
paragraph
The correct answer is: (d) The temperature is kept at
paragraph
9. If 60g of M combines with 24g of oxygen, what would the empirical formula of the oxide be? [O = 16.0, M = 120]
paragraph
(a) MO
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is:
paragraph
10. The products of the electrolysis of dilute sodium chloride using carbon electrodes are
paragraph
(a) Chlorine and sodium
paragraph
(b) Oxygen and hydrogen
paragraph
(c) Chlorine and hydrogen
paragraph
(d) Hydrogen and oxygen
paragraph
The correct answer is: (b) Oxygen and hydrogen
paragraph
WASSCE/WAEC Chemistry (2022) Questions and Answers 11 - 20
paragraph
11. Determine the quantity of electricity used when a current of 0.20 amperes is passed through an electrolytic cell for 60 minutes
paragraph
(a) 12
paragraph
(b) 120
paragraph
(c) 360
paragraph
(d) 720
paragraph
The correct answer is: (d) 720
paragraph
12. Oxochlorate (I) acid is used as a bleaching agent because it is
paragraph
(a) A weak acid
paragraph
(a) A reducing agent
paragraph
(c) An oxidizing agent
paragraph
(d) A strong acidz
paragraph
The correct answer is: (c) An oxidizing agent
paragraph
13. The IUPAC name for is
paragraph
(a) 2, 4-dimethyl-3-chlorohexane
paragraph
(b) 3, 5-dimethyl-4-chlorohexane
paragraph
(c) 4-chloro-3, 5-dimethylhexane
paragraph
(d) 3-chloro-2, 4-dimethylhexane
paragraph
The correct answer is: (d) 3-chloro-2, 4-dimethylhexane
paragraph
14. A colourless gas with a pungent smell is evolved when dilute hydrochloric acid is added to a sample of a salt. the gas evolved could turn
paragraph
(a) Acidified solution colourless
paragraph
(b) Acidified
paragraph
(c) solution green
paragraph
(d) paper black
paragraph
The correct answer is: (d) paper black
paragraph
15. If 5.0g of marble reacts with hydrochloric acid, which of the following combinations has the fastet reaction rate?
paragraph
(a) Marble chips and 2.0 mol
paragraph
(b) Marble chips and 2.5 mol
paragraph
(c) Powdered mmarble and 2.5 mol
paragraph
(d) Powdered marble and 2.0 mol
paragraph
The correct answer is: (c) Powdered mmarble and 2.5 mol
paragraph
16. Increasing the temperature generally
paragraph
(a) Decreases the solubility of a solid in a liquid but increases the solubility of a gas in a liquid
paragraph
(b) Increases the solubility of a solid in a liquid but decreases the solubility of a gas in a liquid
paragraph
(c) Increases the solubility of both a solid and a gas in a liquid
paragraph
(d) Decreases the solubility of both a solid and a gas in a liquid
paragraph
The correct answer is: (b) Increases the solubility of a solid in a liquid but decreases the solubility of a gas in a liquid
paragraph
17. A white precipitate was formed when was added to an aqueous solution of a salt X. The precipate dissolved in dilute HCL with rapid effervescence. Salt X is likely to contain.
paragraph
(a) ions
paragraph
(b) ions
paragraph
(c) ions
paragraph
(d) ions
paragraph
The correct answer is: (d) ions
paragraph
18. Before a reaction could take place, there should be
paragraph
(a) Ionization of reactant particles
paragraph
(b) Breakage of bonds of products
paragraph
(c) Breakage of bonds of reactants
paragraph
(d) Ionization of product particles
paragraph
The correct answer is: (c) Breakage of bonds of reactants
paragraph
19. Consider the following reaction equation:
paragraph
paragraph
Silicon (IV) oxide is acting as
paragraph
(a) A basic oxide
paragraph
(b) A reducing agent
paragraph
(c) An acidic oxide
paragraph
(d) An oxidizing agent
paragraph
The correct answer is: (c) An acidic oxide
paragraph
20. Which of the following acids would form normal salt only?
paragraph
(a) Tetraoxosulphate (VI) acid
paragraph
(b) Trioxonitrate (V) acid
paragraph
(c) Tetraoxosulphate (V) acid
paragraph
(d) Trioxosulphate (IV) acid
paragraph
The correct answer is: (b) Trioxonitrate (V) acid
paragraph
WASSCE/WAEC Chemistry (2022) Questions and Answers 21 - 30
paragraph
21. What is the partial pressure of oxygen at s.t.p in a gaseous mixture containging of oxygen and
paragraph
(a) 1.0 atm
paragraph
(b) 1.0 atm
paragraph
(c) 9.0 atm
paragraph
(d) 0.9 atm
paragraph
The correct answer is: (b) 1.0 atm
paragraph
22. Graphite is used as a dry lubricant due to the presence of
paragraph
(a) Mobile electrons
paragraph
(b) Free electrons
paragraph
(c) Octahedral structures
paragraph
(d) Layered structures
paragraph
The correct answer is: (d) Layered structures
paragraph
23. Which of the following gases is alkaline?
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (c)
paragraph
24. Which of the following statements about an equilibrium system is correct?
paragraph
(a) Forward and backward reactions occur at the same time
paragraph
(b) The concentrations of reactants must equal that of the products.
paragraph
(c) The concentrations of reactants and products can be changed by adding a catalyst
paragraph
(d) The concentrations of reactants and products are not affected by a change in temperature
paragraph
The correct answer is: (a) Forward and backward reactions occur at the same time
paragraph
25. Consider the following reaction equation:
paragraph
paragraph
Increasing the temperature of the reaction would:
paragraph
(a) Shift the equilibrium to the right
paragraph
(b) Increase the yield of ammonia
paragraph
(c) Decrease the amount of hydrogen
paragraph
(d) Decrease the yield of ammonia
paragraph
The correct answer is: (d) Decrease the yield of ammonia
paragraph
26. When air in a syringe is compressed such that there is no change in temperature, the
paragraph
(a) Air liquefies
paragraph
(b) Pressure increases
paragraph
(c) Intermolecular space increases
paragraph
(d) Density decreases
paragraph
The correct answer is: (b) Pressure increases
paragraph
27. Which of the following statements about liquids is/are true? I Liquids maintain their volume at constant temperature II. Liquids have fixed shape III. Liquids do not diffuse IV. Change in pressure affects volume of liquids.
paragraph
(a) I only
paragraph
(b) IV only
paragraph
(c) I and IV only
paragraph
(d) II and III only
paragraph
The correct answer is: (a) I only
paragraph
28. A hydrogen chloride gas eacted with oxygen gas to yield water and chlorine gas. The mole ratio of the hydrogen chloride gas to water is
paragraph
(a) 1:3
paragraph
(b) 2:1
paragraph
(c) 3:1
paragraph
(d) 4:1
paragraph
The correct answer is: (b) 2:1
paragraph
29. What number of moles of oxygen would exert a pressure of 10 atm at 320 K in an cylinder? [R = 0.082 atm ]
paragraph
(a) 0.32
paragraph
(b) 1.52
paragraph
(c) 3.13
paragraph
(d) 31.25
paragraph
The correct answer is: (c) 3.13
paragraph
30. If of saturated solution of at
paragraph
(a) 1.0 mol
paragraph
(b) 1.5 mol
paragraph
(c) 2.0 mol
paragraph
(d) 5.0 mol
paragraph
The correct answer is: (a) 1.0 mol
paragraph
WASSCE/WAEC Chemistry (2022) Questions and Answers 31 - 40
paragraph
31. Which of the following elements would displace copper from a solutionn of copper ions?
paragraph
(a) Silver
paragraph
(b) Gold
paragraph
(c) Lead
paragraph
(d) Mercury
paragraph
The correct answer is: (c) Lead
paragraph
32. What is the percentage composition of carbon in ? [Ca = 40.0, O = 16.0, C = 12.0, H = 1.0]
paragraph
(a) 22.2%
paragraph
(b) 14.8%
paragraph
(c) 1.4%
paragraph
(d) 3.7%
paragraph
The correct answer is: (b) 14.8%
paragraph
33. Which of the following bond types is intermolecular?
paragraph
(a) Covalent bond
paragraph
(b) Hydrogen bond
paragraph
(c) Ionic bond
paragraph
(d) metallic bond
paragraph
The correct answer is: (b) Hydrogen bond
paragraph
34. The maximum number of covalent bonds formed by nitrogen is
paragraph
(a) 1
paragraph
(b) 2
paragraph
(c) 3
paragraph
(d) 4
paragraph
The correct answer is: (c) 3
paragraph
35. The IUPAC name of the compound
paragraph
(a) 2-methyl but-1 ent
paragraph
(b) 2-methyl but-2 ene
paragraph
(c) 3-methyl but-1-ene
paragraph
(d) 3-methyl but-2-ene
paragraph
The correct answer is: (c) 3-methyl but-1-ene
paragraph
36. Ionization energy increases across the period in the periodic table because
paragraph
(a) Atomic number increases
paragraph
(b) Effective nuclear charge increases
paragraph
(c) Mass number decreases
paragraph
(d) Screening effect decreases
paragraph
The correct answer is: (b) Effective nuclear charge increases
paragraph
37. Which of the following properties indicate that an element is a metal. it (I) reacts with oxygen to form an acidic oxide (II) forms ionic chlorides (III) has variable oxidation states (IV) Displaces hydrogen from dilute HCL?
paragraph
(a) I and III only
paragraph
(b) I and II only
paragraph
(c) II and IV only
paragraph
(d) I, II, III and IV
paragraph
The correct answer is: (c) II and IV only
paragraph
38. The electron configuration of carbon atom in its excited state is
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (c)
paragraph
39. An oxide has the following properties. (I) is a white powder (II) Reacts with HCL (III) Reacts with NaOH (IV) is insoluble in water. The oxide is
paragraph
(a) Alkaline
paragraph
(b) Amphoteric
paragraph
(c) Acidic
paragraph
(d) Newtral
paragraph
The correct answer is: (b) Amphoteric
paragraph
**40. Which of the following statements about atoms of a metal is correct? They
paragraph
(a) Readily accept electrons
paragraph
(b) Are soft
paragraph
(c) Are held together by covalent bond
paragraph
(d) Are held together by a sea of electron cloud
paragraph
The correct answer is: (d) Are held together by a sea of electron cloud
paragraph
WASSCE/WAEC Chemistry (2022) Questions and Answers 41 - 50
paragraph
41. Which of the following sketches is a graphical representation of Boyle's law?
paragraph
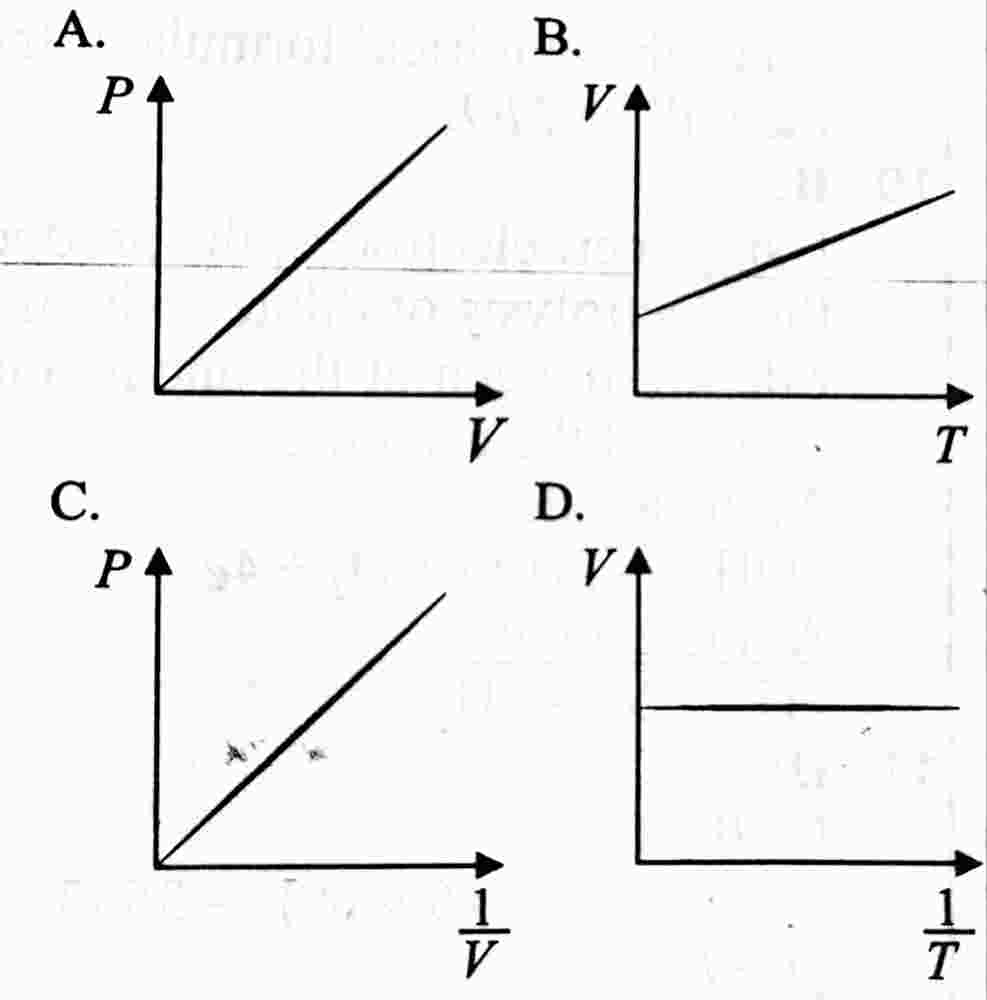
paragraph
The correct answer is: (a) It is a strong oxidizing agent
paragraph
42. The atom with the electron configuration
paragraph
(a) Period 4, p-block
paragraph
(b) Period 3, p-block
paragraph
(c) Period 4, d-block
paragraph
(d) Period 3, d-block
paragraph
The correct answer is: (a) Period 4, p-block
paragraph
43. Electropositivity of elements acros the periodic table normally
paragraph
(a) Remains constant down the group
paragraph
(b) Increases across the period
paragraph
(c) Decreases across the period
paragraph
(d) Decrease down the group
paragraph
The correct answer is: (c) Decreases across the period
paragraph
44. Consider the following table:
paragraph
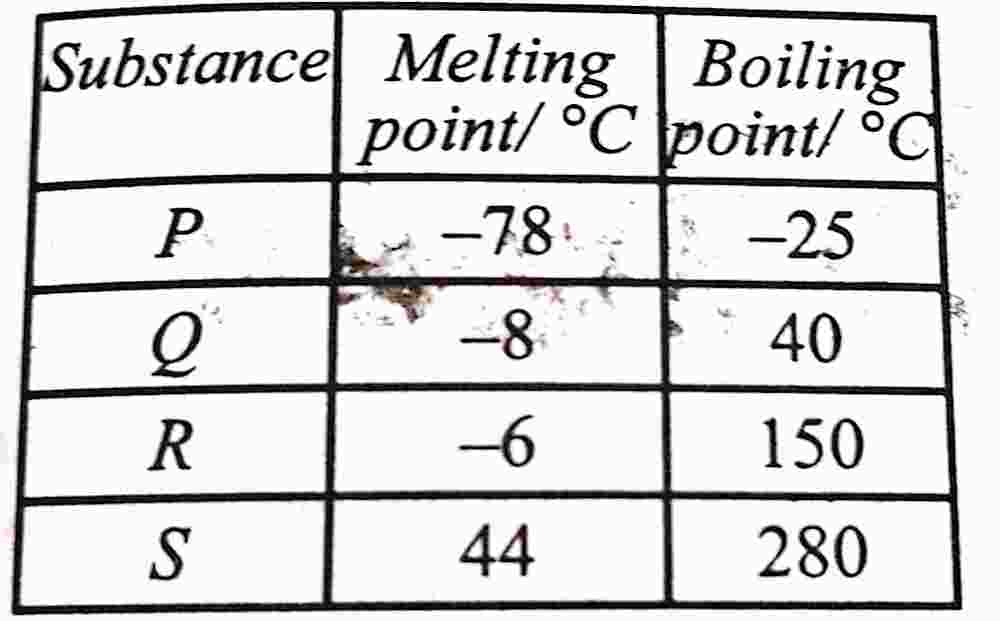
paragraph
Which of the substances is a liquid at room temperature and rapidly evaporates on exposure to air?
paragraph
(a) P
paragraph
(b) Q
paragraph
(c) R
paragraph
(d) S
paragraph
The correct answer is: (b) Q
paragraph
45. Which of the following oxide is amphoteric?
paragraph
(a) Carbon (II) oxide
paragraph
(b) Nitrogen (IV) oxide
paragraph
(c) Lead (II) oxide
paragraph
(d) Calcium oxide
paragraph
The correct answer is: (c) Lead (II) oxide
paragraph
46. Which of the following processes occur during fractional distillation of petroleum?
paragraph
(a) Condensation and diffusion
paragraph
(b) Diffusion and evaporation
paragraph
(c) Diffusion and sublimation
paragraph
(d) Evaporation and condensation
paragraph
The correct answer is: (d) Evaporation and condensation
paragraph
**47. If the molar mass of , determine the relative atomic mass of X. [H = 1.0, C = 12.0, O = 16.0]
paragraph
(a) Energy is released when liquids change to solids
paragraph
(b) Particles move faster in the gaseous state than in the liquid state
paragraph
(c) Carbon atoms in gaseous methane are further apart than those in solid diamond
paragraph
(d) There is large decrease in the volume of a solid metal when pressure is applied to it
paragraph
The correct answer is: (d) There is large decrease in the volume of a solid metal when pressure is applied to it
paragraph
48. Charcoal is used in the decolourization of sugar because of its
paragraph
(a) Absorption property
paragraph
(b) Amorphous property
paragraph
(c) Oxidizing property
paragraph
(d) Adsorption property
paragraph
The correct answer is: (d) Adsorption property
paragraph
49. The first definition of an element was made by:
paragraph
(a) A.J.Dalton
paragraph
(b) B.A.Lavoisier
paragraph
(c) C. R. Boyle
paragraph
(d) D. J.J.Thomson
paragraph
The correct answer is: (c) C. R. Boyle
paragraph
50. Which of the following scientists formulated the law of conservation of mass?
paragraph
(a) A. Lavoisier
paragraph
(b) B. J.Dalton
paragraph
(c) C. R.Boyle
paragraph
(d) D. J.Proust
paragraph
The correct answer is: (a) A. Lavoisier
paragraph
Download WASSCE/WAEC Chemistry pdF (2022) Paper 2 (essay questions and answer 1 - 5)
paragraph
We have provided a pdf where you can practice wassce Paper 2 (essay questions) for Chemistry 2022. To download the
pdf right away,
paragraph
Download WASSCE/WAEC Chemistry pdf (2023) Paper 3 (Practical questions and answer 1 - 3)
paragraph
We have also provided a pdf where you can practice wassce Paper 3 (practical) for chemistry 2022. To download the
pdf right away,
paragraph
Without doubt I believe you are happy with the questions and answers on WASSCE/WAEC Chemistry 2022 you
saw above. We have compiled this to ensure students have access to resources that can help them
blast their WASSCE exam. If you have any comment or you observe any flaw do well to leave a
comment in the comment-box below and we will get back to you
paragraph
I recommend you check my article on the following:
paragraph
- WASSCE/WAEC past questions and answers for CHEMISTRY-2023
paragraph
This is all we can take on “WASSCE objective, essay, and practical past questions and answers for (CHEMISTRY 2022)“.
paragraph
