WASSCE objective, essay, and practical past questions and answer (CHEMISTRY 2023)
paragraph
I warmly welcome you to Poscholars webite. First of all, I deem it fit to congratulate you in advance because we believe that
for you to stumble on this post, it simply means you are ready to learn. In this post, we have compiled past questions
and answers for wassce/waec 2023 Chemistry objective, the paper 2 and the paper 3(practical) have been compiled in form of pdf. I encourage
you to go through this post thoroughly as it will be of immense benefit to you.
paragraph
You can study the 50 (fifty) objectives, theory and practical WASSCE physics past questions and answer for 2023 by checking
the content below or using the table of content below to navigate to the number of your choice
paragraph
Table of Contents
- WASSCE/WAEC Chemistry (2023) Questions and Answers 1 - 10
- WASSCE/WAEC Chemistry (2023) Questions and Answers 11 - 20
- WASSCE/WAEC Chemistry (2023) Questions and Answers 21 - 30
- WASSCE/WAEC Chemistry (2023) Questions and Answers 31 - 40
- WASSCE/WAEC Chemistry (2023) Questions and Answers 41 - 50
- Download WASSCE/WAEC Chemistry pdF (2023) Paper 2 (essay questions and answer 1 - 5)
- Download WASSCE/WAEC Chemistry pdf (2023) Paper 3 (Practical questions and answer 1 - 3)
paragraph
WASSCE/WAEC Chemistry (2023) Questions and Answers 1 - 10
paragraph
1. Which of the following laws or theory cannot be explained by the application of the kinetic theory of gases?
paragraph
(a) Dalton's atomic theory
paragraph
(b) Charles' law
paragraph
(c) Gay-lussac's law
paragraph
(d) Boyle's law
paragraph
The correct answer is: (a) Dalton's atomic theory
paragraph
2. The primary component of natural gas is
paragraph
(a) Butane
paragraph
(b) Ethane
paragraph
(c) Methane
paragraph
(d) Propane
paragraph
The correct answer is: (c) Methane
paragraph
3. Thermal cracking of alkanes usually
paragraph
(a) Involves decomposition
paragraph
(b) Is an exothermic process
paragraph
(c) Produces only small alkanes
paragraph
(d) Requires hydrogen
paragraph
The correct answer is: (a) Involves decomposition
paragraph
4. Carbon is deposited in the exhaust pipes of cars because of
paragraph
(a) Contamination of petrol with diesel
paragraph
(b) Presence of carbon petrol
paragraph
(c) Presence of additives in petrol
paragraph
(d) Incomplete combustion of petrol
paragraph
The correct answer is: (d) Incomplete combustion of petrol
paragraph
5. How many moles of copper would be deposited by passing 1 Faraday of electricity through a , solution?
paragraph
(a) 2
paragraph
(b) 1
paragraph
(c) 0.5
paragraph
(d) 0.25
paragraph
The correct answer is: (c) 0.5
paragraph
6. Alkenes can be manufactured by
paragraph
(a) Addition fo hydrogen to unsaturated vegetable oils
paragraph
(b) The cracking of hydrocarbons
paragraph
(c) Polymerization reactions
paragraph
(d) The combustion of alkanes
paragraph
The correct answer is: (b) The cracking of hydrocarbons
paragraph
7. Petrochemistry is an example of
paragraph
(a) Pure chemistry
paragraph
(b) Applied chemistry
paragraph
(c) Biochemistry
paragraph
(d) Environmental chemistry
paragraph
The correct answer is: (b) Applied chemistry
paragraph
8. Species that occur in a reaction pathway but not in the overall reaction arc known as
paragraph
(a) Products
paragraph
(b) Inhibitors
paragraph
(c) Reactants
paragraph
(d) Intermediates
paragraph
The correct answer is: (d) Intermediates
paragraph
9. Which of the following statements is correct?
paragraph
(a) An alkane with 49 carbon atoms contain 100 hydrogen atoms
paragraph
(b) Addition reactions occur between alkanes and chlorine
paragraph
(c) Butane has a lower boiling point than propane
paragraph
(d) Pentane has five isomers
paragraph
The correct answer is: (a) An alkane with 49 carbon atoms contain 100 hydrogen atoms
paragraph
10. The best indicator to use for the titratiojn of ethanoic acid with sodium hydroxide is
paragraph
(a) Methyl red
paragraph
(b) Methyl orange
paragraph
(c) Phenolphthalein
paragraph
(d) Screened methyl orange
paragraph
The correct answer is: (c) Phenolphthalein
paragraph
WASSCE/WAEC Chemistry (2023) Questions and Answers 11 - 20
paragraph
11. The reduction half equation half equation of the following reaction is:
paragraph
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (b)
paragraph
12. If of a saturated solution of sodium tetraoxosulphate (VI) at contains 10.5g of the salt, what would be its solubility at this temperature? []
paragraph
(a)
paragraph
(a)
paragraph
(a)
paragraph
(a)
paragraph
The correct answer is: (a)
paragraph
13. An example of a crystalline substance that does not possess water of crystallization is
paragraph
(a) Potassium trioxonitrate
paragraph
(b) Sodium trioxocarbonate (IV)
paragraph
(c) Iron (II) Tetraoxosulphate (VI)
paragraph
(d) Sodium tetraoxosulphate (VI)
paragraph
The correct answer is: (a) Potassium trioxonitrate
paragraph
14. The salt solution formed from a reaction between ethanoic acid and sodium hydroxide solution would be
paragraph
(a) Basic
paragraph
(b) Acidic
paragraph
(c) Neutral
paragraph
(d) Amphoteric
paragraph
The correct answer is: (a) Basic
paragraph
15. Which of the following reactions represent the hydrolysis of an alkanoate?
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (c)
paragraph
The correct answer is: (b) Beat
paragraph
16. Which of the following statement about the collision theory is correct?
paragraph
(a) All collisions bring about reactions
paragraph
(b) Rate of reaction is proportional to the number of effective collisions
paragraph
(c) Collision of molecules will split the molecules to react
paragraph
(d) Ineffective collision brings about chemical reaction.
paragraph
The correct answer is: (b) Rate of reaction is proportional to the number of effective collisions
paragraph
17. Why are not suitable for drying ammonia gas? They
paragraph
(a) Are corrosive
paragraph
(b) Are poisonous
paragraph
(c) React with the gas
paragraph
(d) Pollute the gas
paragraph
The correct answer is: (c) React with the gas
paragraph
18. Which of the following equations does not illustrate correctly one of the reactions of chlorine?
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (c)
paragraph
19. How many unpaired electrons are present in
paragraph
(a) 2
paragraph
(b) 3
paragraph
(c) 4
paragraph
(d) 5
paragraph
The correct answer is: (d) 5
paragraph
20. Going down group II in the periodic table normally
paragraph
(a) Shielding effect decreases
paragraph
(b) Melting point increases
paragraph
(c) Ionization energy increases
paragraph
(d) Electronegativity increases
paragraph
The correct answer is: None of the option is correct
paragraph
WASSCE/WAEC Chemistry (2023) Questions and Answers 21 - 30
paragraph
21. Which of the following elements has its valence electrons in the S-orbital
paragraph
(a) Sodium
paragraph
(b) Carbon
paragraph
(c) Phosphorus
paragraph
(d) Aluminium
paragraph
The correct answer is: (a) Sodium
paragraph
22. The periodic property that is used to determine whether a covalent molecule is polar or not is
paragraph
(a) Atomic radius
paragraph
(b) Electron affinity
paragraph
(c) Electronegativity
paragraph
(d) Ionization energy
paragraph
The correct answer is: (c) Electronegativity
paragraph
23. The following steps are scientific methods except
paragraph
(a) Analysis
paragraph
(b) Open-mindedness
paragraph
(c) Experimentation
paragraph
(d) Problem identification
paragraph
The correct answer is: (b) Open-mindedness
paragraph
24. Isoelectronic species have the same number of
paragraph
(a) Electrons
paragraph
(b) Neutrons
paragraph
(c) Protons
paragraph
(d) Ions
paragraph
The correct answer is: (a) Electrons
paragraph
25. An element X, has two isotopes, with relative abundance of 60% and 40% respectively. The relative atomic mass of X is
paragraph
(a) 65.00
paragraph
(b) 65.40
paragraph
(c) 65.50
paragraph
(d) 66.00
paragraph
The correct answer is: (b) 65.40
paragraph
26. The pair of compounds that belongs to the same homologous series is
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (d)
paragraph
27. A consequence of global warming is
paragraph
(a) Flooding
paragraph
(b) Water pollution
paragraph
(c) Air pollution
paragraph
(d) High humidity
paragraph
The correct answer is: (a) Flooding
paragraph
28. Which of the following gases has the lowest rate of diffusion? [H = 1.0, C = 12.0, N = 14.0, O = 16.0]
paragraph
(a) Nitrogen
paragraph
(b) Ammonia
paragraph
(c) Oxygen
paragraph
(d) Methane
paragraph
The correct answer is: (c) Oxygen
paragraph
29. The gas that is less dense than air is
paragraph
(a) Carbon (IV) oxide
paragraph
(b) Nitrogen
paragraph
(c) Chlorine
paragraph
(d) Oxygen
paragraph
The correct answer is: (b) Nitrogen
paragraph
30. Which of the following equimolar solutions has the highest conductivity?
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (b)
paragraph
WASSCE/WAEC Chemistry (2023) Questions and Answers 31 - 40
paragraph
31. What takes place at the cathode during electrolysis?
paragraph
(a) Anions lose electrons
paragraph
(b) Anions are oxidized
paragraph
(c) Cations are discharged
paragraph
(d) Cations are oxidized
paragraph
The correct answer is: (c) Cations are discharged
paragraph
32. How many grammes of would be needed to produce ? [NaOH = 40.0]
paragraph
(a) 0.02g
paragraph
(b) 0.80g
paragraph
(c) 20.0g
paragraph
(d) 800.0g
paragraph
The correct answer is: (b) 0.80g
paragraph
33. The formation of a bond between hydrogen and a highly electronegaive atom results in
paragraph
(a) Polarity
paragraph
(b) Dipole
paragraph
(c) Metallic bond
paragraph
(d) Elecrovalent bond
paragraph
The correct answer is: (a) Polarity
paragraph
34. The molecules that has a non-polar covalent bond is
paragraph
(a)
paragraph
(b) HCl
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is:
paragraph
35. Which of the elements in the table below would react more readily with chlorine?
paragraph
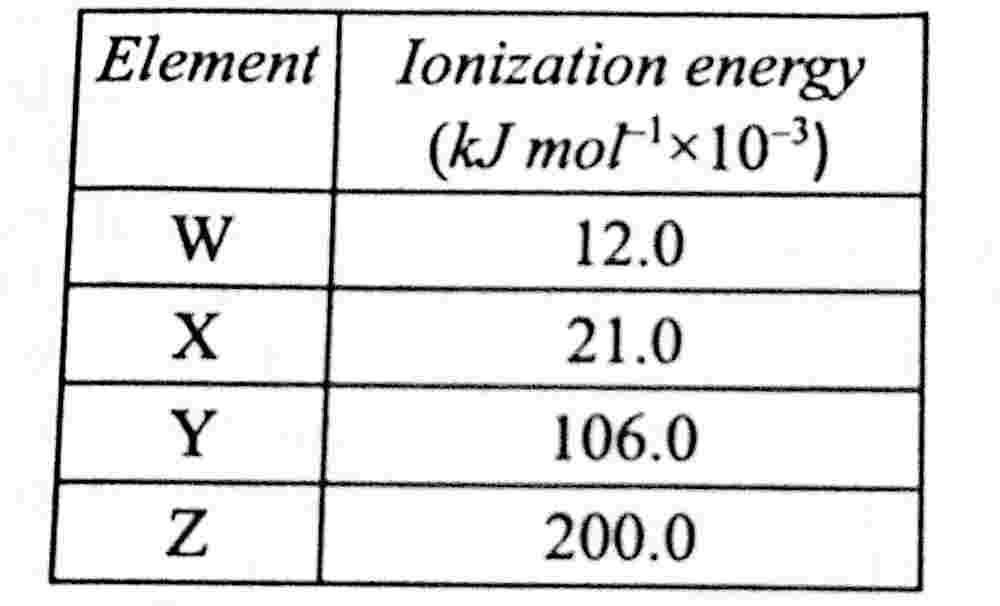
paragraph
(a) W and X only
paragraph
(b) W and Z only
paragraph
(c) X and Z only
paragraph
(d) Y and Z only
paragraph
The correct answer is: (a) W and X only
paragraph
36. The relative molar mass of a gaseous hydrocarbon is 30. Determine it vapour density
paragraph
(a) 15
paragraph
(b) 30
paragraph
(c) 60
paragraph
(d) 45
paragraph
The correct answer is: (a) 15
paragraph
37. Consider the following reaction equation:
paragraph
paragraph
Which of the following statements about the reaction is correct?
paragraph
(a) The reaction is exothermic
paragraph
(b) The reaction container would feel warm
paragraph
(c) 198kJ of energy is given off
paragraph
(d) 198kJ of energy is absorbed
paragraph
The correct answer is: (d) 198kJ of energy is absorbed
paragraph
38. Which of the following properties does not give evidence of the kinetic theory of matter?
paragraph
(a) Evaporation
paragraph
(b) Diffusion
paragraph
(c) Polymerization
paragraph
(d) Melting
paragraph
The correct answer is: (c) Polymerization
paragraph
39. A compound that could be dried by using conc. tetraoxosulphate (VI) acid and not by calcium oxide is likely to be
paragraph
(a) An alkaline gas
paragraph
(b) A neutral salt
paragraph
(c) A deliquescent salt
paragraph
(d) An acid anhydride
paragraph
The correct answer is: (d) An acid anhydride
paragraph
**40. Positive ions in a sea of electrons are found in
paragraph
(a) Covalent bonds
paragraph
(b) Dative bonds
paragraph
(c) Ionic bonds
paragraph
(d) Metallic bonds
paragraph
The correct answer is: (d) Metallic bonds
paragraph
WASSCE/WAEC Chemistry (2023) Questions and Answers 41 - 50
paragraph
41. Dilute trioxonitrate (V) acid does not produce hydrogen when it reacts with metals because
paragraph
(a) It is a strong oxidizing agent
paragraph
(b) It reacts with the product
paragraph
(c) There is no visible reaction
paragraph
(d) It is highly corrosive
paragraph
The correct answer is: (a) It is a strong oxidizing agent
paragraph
42. How many moles are there in 3.0g of ? [O = 16]
paragraph
(a) 0.0093
paragraph
(b) 0.0930
paragraph
(c) 0.6250
paragraph
(d) 0.0625
paragraph
The correct answer is: (d) 0.0625
paragraph
43. Copper (II) ions are able to participate in co-ordinate covalent bonding because they
paragraph
(a) Are coloured
paragraph
(b) Have unpaired electrons
paragraph
(c) Have vacant d-orbital
paragraph
(d) Are positively charged
paragraph
The correct answer is: (c) Have vacant d-orbital
paragraph
44. Determine the volume of 0.100 mol of HCl in 0.250 mol of solution
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (d)
paragraph
45. Which of the statements about gases is not correct?
paragraph
(a) The total kinetic energy of the gas is not affected by collision
paragraph
(b) Molecules of the gas are in constant motion
paragraph
(c) Gases have low densities compared to solids and liquids of equal mass
paragraph
(d) Gases are highly soluble in water at high temperatures
paragraph
The correct answer is: (d) Gases are highly soluble in water at high temperatures
paragraph
46. Arrange the following compounds in decreasing order of their boiling points
paragraph
paragraph
(a)
paragraph
(b)
paragraph
(c)
paragraph
(d)
paragraph
The correct answer is: (c)
paragraph
47. The following statements are correct except
paragraph
(a) Energy is released when liquids change to solids
paragraph
(b) Particles move faster in the gaseous state than in the liquid state
paragraph
(c) Carbon atoms in gaseous methane are further apart than those in solid diamond
paragraph
(d) There is large decrease in the volume of a solid metal when pressure is applied to it
paragraph
The correct answer is: (d) There is large decrease in the volume of a solid metal when pressure is applied to it
paragraph
48. The vapour density of an organic compound with the molecular formula is [H = 1.0, C = 12.0, O = 16.0]
paragraph
(a) 120
paragraph
(b) 65
paragraph
(c) 40
paragraph
(d) 30
paragraph
The correct answer is: (d) 30
paragraph
49. A mixture containing two salts of different solubility can be separated by
paragraph
(a) Chromatography
paragraph
(b) Distillation
paragraph
(c) Crystallization
paragraph
(d) Evaporation
paragraph
The correct answer is: (c) Crystallization
paragraph
50. The separation technique that is suitable for separating iodine from tetrachloromethane is
paragraph
(a) Solvent extraction
paragraph
(b) Fractional distillation
paragraph
(c) Decantation
paragraph
(d) Filtration
paragraph
The correct answer is: (a) Solvent extraction
paragraph
Download WASSCE/WAEC Chemistry pdF (2023) Paper 2 (essay questions and answer 1 - 5)
paragraph
We have provided a pdf where you can practice wassce Paper 2 (essay questions) for Chemistry 2023. To download the
pdf right away,
paragraph
Download WASSCE/WAEC Chemistry pdf (2023) Paper 3 (Practical questions and answer 1 - 3)
paragraph
We have also provided a pdf where you can practice wassce Paper 3 (practical) for Chemistry 2023. To download the
pdf right away,
paragraph
Without doubt I believe you are happy with the questions and answers on WASSCE/WAEC Chemistry 2023 you
saw above. We have compiled this to ensure students have access to resources that can help them
blast their WASSCE exam. If you have any comment or you observe any flaw do well to leave a
comment in the comment-box below and we will get back to you
paragraph
I recommend you check my article on the following:
paragraph
- WASSCE/WAEC past questions and answers for CHEMISTRY-2022
paragraph
This is all we can take on “WASSCE objective, essay, and practical past questions and answer for (CHEMISTRY 2023)“.
paragraph
